The
"stickmen" of L S Lowry, populating his paintings of Northern
urban landscapes, walk hunched around: their lungs damaged by the smoke
and fumes of a coal-burning industrial revolution. It is universally
believed that the coal reserves on which our prosperity found its
origins are likely to endure for hundreds of years and that its only
drawback is the pollution arising from its combustion. .
An awareness of rising levels of carbon dioxide from man's industrial activity based on a rising combustion of fossil fuels has triggered the climate change politically-charged 'potboiler'. This is now cast as the world's main problem—as carbon dioxide goes "across national boundaries"—its rising concentration in the atmosphere is believed to be causing global warming, which if allowed to continue will, by the end of the century, lead to catastrophe.
 The
expanding life-style it fostered for the industrialists was also
disadvantageous for many by the effect its mining had on local
environments, such as the pit heaps from underground mining and later
the devastation of open pit extraction. The atmosphere found little
relief until The Clean Air Act was applied in 1956, which dealt with
emissions of "dark smoke, grit and dust".
The
expanding life-style it fostered for the industrialists was also
disadvantageous for many by the effect its mining had on local
environments, such as the pit heaps from underground mining and later
the devastation of open pit extraction. The atmosphere found little
relief until The Clean Air Act was applied in 1956, which dealt with
emissions of "dark smoke, grit and dust".
Apart from being composed of mainly carbon, coal contains various amounts of sulphur and in the 1960's, the concern was the production of "acid rain" from the sulphur dioxide emissions, which led to forest damage within and beyond the boundaries of the user country. High chimneys reduced the local concentrations and consequent damage to people, plants and buildings, but spread the contamination far and wide. [1]
In 1971, Sweden complained to the United Nations [2] about the impact on its environment of sulphur in air and its precipitation, affecting the health of its citizens, animals and plants. It monitored the acidification of its soils, forests and lakes from emissions arising downwind in North-West Europe with a large contribution from UK industries.
Thirty years later, the concern is the release of carbon dioxide. A relatively inert gas expended by all animals, it isabsorbed by plants, which balancing effect once held concentrations in the atmosphere at relatively stable levels. An awareness of rising levels of carbon dioxide from man's industrial activity based on a rising combustion of fossil fuels has triggered the climate change politically-charged 'potboiler'. This is now cast as the world's main problem—as carbon dioxide goes "across national boundaries"—its rising concentration in the atmosphere is believed to be causing global warming, which if allowed to continue will, by the end of the century, lead to catastrophe.
Around 60% by weight of coal is carbon, with varying amounts of moisture and non-combustibles resulting in ash, so that its combustion results mainly in emissions of carbon dioxide. High temperature operation "fixes" some atmospheric nitrogen as oxides which together with sulphur oxides and ash particulates contribute to local air quality problems. There are other contaminants released, such as mercury and even a modicum of radioactivity.
For this reason flue gas treatment technologies are applied, but the removal of carbon dioxide and its sequestration present new challenges. Recourse to the use of more coal in substitution for oil and gas fuels will rely on "clean coal technologies" (CCT) to avoid a contribution to climate change.
MIT has published an exhaustive report "The Future of Coal" which examines the various technologies
- for carbon capture and sequestration and
- those for the production of alternative fuels and chemicals. See [3]
There is an economic penalty in adopting clean coal technologies as more coal is needed to provide the additional energy required by the ancillary processes for the separation and sequestration of the carbon dioxide. The MIT report provides a useful set of process flow charts with mass balances for the evaluation of the clean-up methods.
There's always coal?
The MIT report begins in its Executive Summary with the premise "… coal use will increase under any foreseeable scenario because it is cheap and abundant." Commentators refer to "hundreds of years" of coal, so it is necessary to demythologise this sub-conscious, comfortable view of future security. MIT notes that coal is not a single material—its composition and properties vary considerably.
 Reference
to BP's Statistical Review of World Energy 2006 [4] reveals proved coal
reserves totalling 909 billion tonnes, which at the current rate of
production of 5.8 billion tonnes per annum will presumably last 155
years. The coal is rated under two categories, 478 billion tonnes of
'anthracites and bituminous' and 430 billion tonnes of 'sub-bituminous
and lignite'. Anyone visiting East Germany before 1990, will have smelt
the sulphurous tang of the lignite (heating value 15-19 MJ/kg)
"brown coal" briquettes, which kept the populace warm.
Sub-bituminous coal (20-28 MJ/kg) is little better than lignite,
containing 20% to 30% moisture by weight. Bituminous coal (27 MJ/kg)
contains bitumen, while the most desired is anthracite (27-30 MJ/kg).
Reference
to BP's Statistical Review of World Energy 2006 [4] reveals proved coal
reserves totalling 909 billion tonnes, which at the current rate of
production of 5.8 billion tonnes per annum will presumably last 155
years. The coal is rated under two categories, 478 billion tonnes of
'anthracites and bituminous' and 430 billion tonnes of 'sub-bituminous
and lignite'. Anyone visiting East Germany before 1990, will have smelt
the sulphurous tang of the lignite (heating value 15-19 MJ/kg)
"brown coal" briquettes, which kept the populace warm.
Sub-bituminous coal (20-28 MJ/kg) is little better than lignite,
containing 20% to 30% moisture by weight. Bituminous coal (27 MJ/kg)
contains bitumen, while the most desired is anthracite (27-30 MJ/kg).
On a calorific or heating value basis a tonne of anthracite and bituminous coal is equivalent to 0.67 tonnes oil equivalent or around 5 barrels oil equivalent (boe), whereas this drops to 0.33 tonnes or 2.5 boe for sub-bituminous and lignite. This means that the coal reserves equate to around 3,500 gigabarrels oil equivalent (Gboe), compared with the 1,200 Gb of oil reserves at the end of 2005.
The barrels of oil equivalent concept needs examination. It is based on the respective calorific values (Higher and lower heating values, HHV and LHV) of oil and of the coal type. The straightforward combustion in a boiler allows an equivalent heating value for oil, gas and coal to be based on calorific values, but in their other usages, the equivalents need to be measured in terms of their applicability. Liquid fuels for transport, viz., petrol, diesel and jet fuel can be readily produced in an oil refinery— whereas although the same can be produced using gas-to-liquids processes (GTL) and coal-to-liquids (CTL) processes—the low thermal efficiency of the conversion reduces the comparative oil equivalents.
This means that as oil production enters its decline having passed its peak, substitute liquid fuels made from natural gas and coal require more than the simple calorific oil equivalent amounts to achieve the same usefulness. This, in turn, means that the reserves of gas and coal are used up faster than might otherwise be construed from their oil equivalents and the production peaks in gas and coal are brought forward.
As the MIT report shows, the adoption of carbon capture and sequestration (CCS) in electricity generation requires more gas or coal input for the same electrical output compared with simply burning coal and treating the flue gases for particulate emissions.
So a combination of CTL and CCS would lead to a more rapid exhaustion of coal reserves; while a reversion to coal-fired generation instead of combined heating and generation with gas, together with global economic growth (or business as usual) will eat into the 155 years assumed in the BP Statistical Review. Taking all the above factors into account, a peak in coal production could well occur around 2040-50. The Energy Watch Group believes this could occur even sooner by 2025. [5]
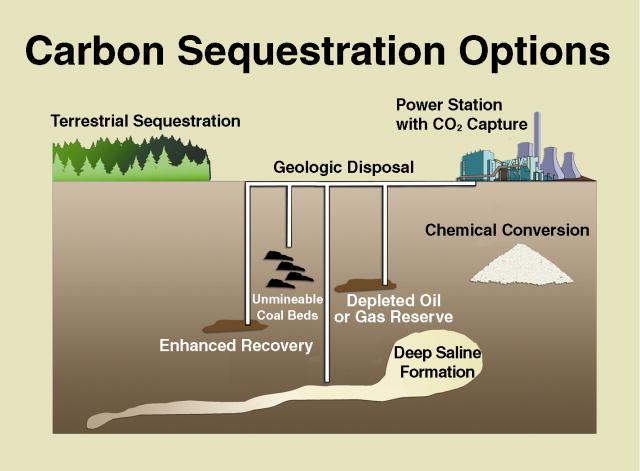
Carbon capture and sequestration (CCS)
MIT considered four main types of coal electricity generation technologies, in three of them the coal is pulverised to a talcum-powder fineness for injection into the boiler with combustion air. The flue gas is then cleaned up to remove particulates and oxides of nitrogen and sulphur before passing to a CO2 capture unit. Lower coal grades, such as lignite are crushed and mixed with lime in a fluid bed to remove sulphur dioxide, but the CO2 in the flue gases has also to be captured.
The capture unit removes around 90% of the carbon dioxide in the flue gas, which is then cooled, dried and compressed to a supercritical fluid at 150 atmospheres pressure for storage or onward transmission by pipeline.
In the method preferred by MIT, the CO2 in the flue gas is absorbed in an amine, typically monoethanolamine which is a petrochemical made from ammonia and ethylene oxide, in an absorption tower. The absorbed CO2 in the amine solution is then stripped out by heating it with steam, recycling the amine to the absorption tower.
Additional energy is required for the CO2 capture, and its treatment together with that required for its compression. In effect between 27% and 37% more coal for a given electrical output is required. Moreover, the generating station must be sited with reasonable access to a suitable depository for the carbon sequestration or otherwise the additional energy needed to build a long pipeline and transmit the gas through it to the geological storage facility (or to an oil reservoir for boosting its production) will be excessive. If the station is far from the consumers, then additional transmission losses will be a factor.
In any case, the number of suitable locations for carbon sequestration must be few and far between and the availability of the same may well be a limiting factor. There are doubts as to the security of underground cavities. If the CO2 eventually leaks out the whole exercise will be pointless. The most desirable sequestration objects are in oil reservoirs where declining production can be boosted by the injection, but there cannot be many near population centres where the electricity is needed.
The CCS generating station will be 65% more capital intensive with the additional equipment and will be 63% more costly to run with additional coal, supplies of chemicals and water. Taking into account normal day-to-day inefficiencies, it can be assumed that CCS technologies will require 50% more coal than normal and if applied with some universality will bring forward a national coal production peak. Also generating thermal efficiencies will be reduced by around 10%, typically from 35% down to 25%. So carbon capture and sequestration brings with it severe economic and conservation penalties.
Oil, gas and coal reserves and climate change
After oil and natural gas production has passed its peaks in 2010 and 2020, coal will be the fossil fuel of last resort. The universal adoption of carbon capture and sequestration would in effect reduce the remaining extractable coal reserves by a third. An energy-hungry world will not consider a technology with equanimity that eats into its coal reserves with such voracity.

Underground mining is expensive in manpower and casualties, while open-cast mining is primary energy intensive, especially in diesel. A reduction in the usefulness of the coal produced of such a magnitude will find no enthusiastic embrace.
Present value will preside over future concerns about climate change, which will in any case be limited in the second half of the century by fossil fuel exhaustion, which will lead to a worse crisis, viz., world economic collapse.[6] The world needs energy-lean technologies to acquire an energy-lean lifestyle and technologies that require a bigger input for the same output do not fit the specification.
[1] The sulphur dioxide combined with ammonia from decaying vegetation to produce a haze of atmospheric aerosols formed on the nuclei of particulates.
[2] Air pollution across national boundaries. The impact on the environment of sulphur in air and precipitation, Sweden's Royal Ministries of Foreign Affairs and Agriculture, 1971.
[3] MIT's "The Future of Coal" http://web.mit.edu/coal/
[4] BP Statistical Review http://www.bp.com
[6] See "The Limits to Growth" Meadows et al